product
Philips Visions PV 018 Rx digital IVUS catheter
Manufacturer Info
Why to use it
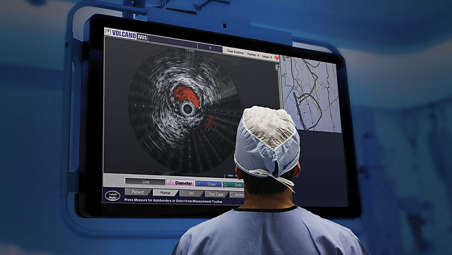
When used in conjunction with conventional angiography, the Visions PV 018 Rx digital IVUS catheter provides additional information on vessel architecture and pathology to aid disease assessment, inform intervention decisions and confirm technical success.
With a 135 cm working length, 24 mm imaging diameter, .018” guide wire compatibility, and blood flow assessment with Chromaflo, the Visions PV 018 Rx digital IVUS catheter allows clinicians to see clearly and treat optimally.
Characteristics
Philips Visions PV IVUS aids in disease assessment
Philips Visions PV IVUS augments angiography by providing precise information on vessel architecture and pathology including: vessel size and percent stenosis; plaque morphology; the significance and presence of dissection; the severity of calcium; and the presence of thrombus.
Philips Visions PV IVUS informs intervention decisions
Philips Visions PV IVUS improves treatment strategy by providing additional information to guide and decide: appropriate angioplasty technique based on true vessel size and lesions length; appropriate device selection based on plaque geometry and morphology and guidewire orientation; and if a patient is at high risk of resttenosis.
Philips Visions PV IVUS confirms technical success
Philips Visions PV IVUS helps confirm the completeness of treatment by assessing and confirming: significant residual narrowing or dissection in the target lesion; the apposition and expansion of stent placement; and whether or not the patient requires a thrombolytic drip.


